News Story
Stroka Takes New Look at Cell Migration

Dr. Kim Stroka, BIOE associate professor and alumna (Ph.D. '11)
University of Maryland Fischell Department of Bioengineering Associate Professor and alum Kimberly Stroka (Ph.D. '11) was awarded a five-year, $1.9 million grant from the National Institutes of Health/National Institute of General Medical Sciences to investigate how a particular protein that is known to control water transport across the cell membrane also influences cell migration in a range of physiological and pathological processes.
In the human body, cells often move from one location to another in response to chemical or mechanical signals. This process – known as cell migration – is critical to our growth to adulthood and our survival; it plays a major role in wound and tissue repair and in facilitating the body’s immune response during an infection.
But, cell migration isn’t always a good thing. In fact, this same movement is what enables cancer cells to break away from a primary site to other parts of the body through a process known as cancer metastasis, or spread. Furthermore, cell migration is involved with numerous other diseases or processes that lead to diseases, from chronic inflammation to endometriosis.
A first step toward understanding what drives cell migration involves exploring how the microenvironments through which cells travel influence their movement.
Cell migration involves trafficking through complex biochemical and physical microenvironments, including those defined as confined spaces. These spaces or channels are tiny, narrow tracks carved out in tissues, along the body’s different anatomical features, or between what are known as endothelial cells as they squeeze into or out of blood vessels.
Recently, Stroka and other scientists have shown that the mechanisms by which cells migrate through these confined microenvironments are likely quite different from the mechanisms used to cross 2D surfaces – such as protein-coated glass coverslips, which scientists in the cell migration field have used as a model system for decades. Recognizing this, researchers have developed many types of engineered devices and models to investigate how cells migrate through confined spaces and through other types of complex 3D environments.
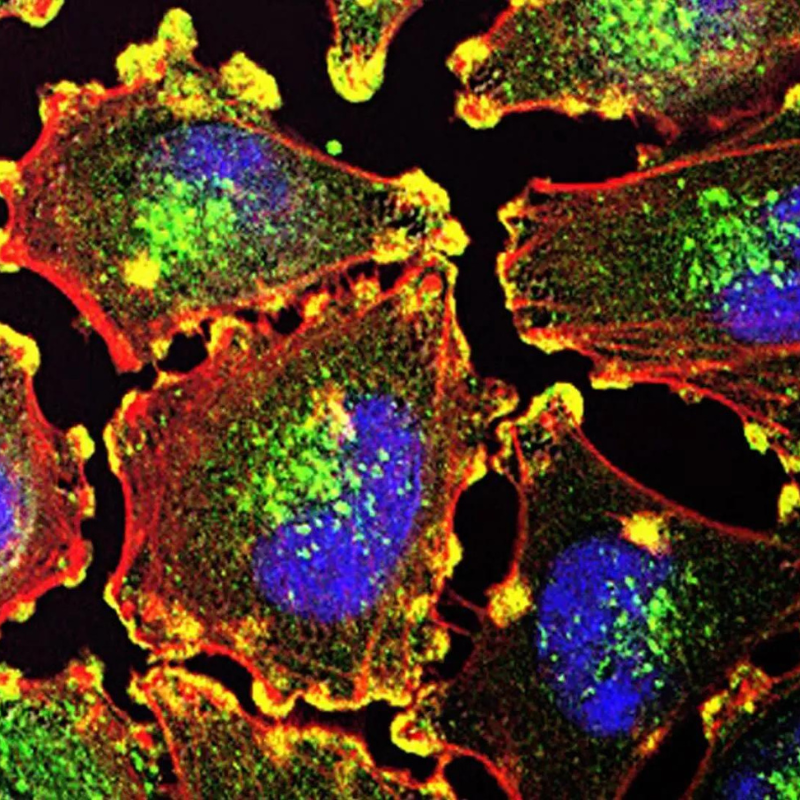
In a previous paper, Dr. Stroka showed that aquaporins (AQPs) – proteins that control water flow across the cell membrane – have an important role in cell migration through these confined spaces. To build on this, Strokaand members of her Cell and Microenvironment Engineering Lab are using engineered cell microenvironments to explore unanswered questions about what microenvironment factors cause AQP expression to change and how these AQPs are actually influencing cell migration.
AQPs are a unique type of protein that selectively permit water flux across the cell membrane and help regulate cell volume. They can be found in certain tissues including in the kidney, brain, and skin. Tissues that house AQPs on the cellular surface can see up to a 50-fold increase in the cell permeability to water when compared with cells that do not express AQPs on their membranes. As such, AQPs play a major role in a variety of physiological processes including fat metabolism, urine concentration, skin moisturization, and brain water homeostasis – a process that helps maintain the blood brain barrier, the network of blood vessels and tissue that protects the brain from harmful toxins and pathogens.
“Interestingly, many types of invasive cells have increased expression of aquaporins,” Stroka said. “However, the field is currently lacking a comprehensive understanding of how aquaporins are actually functioning in cells to influence cell migration. This gap exists in part because of a lack of bioengineering tools, but also because the role of aquaporins in cells seems to be quite complicated and intertwined; aquaporins not only regulate water permeability, but also cell volume, cell biomechanics, and cell signaling. My lab’s goal is to first develop the necessary molecular biology toolset and engineered devices, and then use those to mechanistically study aquaporin function in migratory cells.”
Because AQPs regulate so many cell behaviors, Stroka believes that new insights into how these proteins function could not only shed new light on mechanisms driving normal physiological processes, but also lead to advanced understanding of what drives cells to become invasive in various diseases.
“Aquaporins are a fascinating protein to study because their expression increases in multiple diseases that involve cell invasion into tissues, and they are expressed on the cell surface, meaning that their function may be easily targetable by antibodies or other molecular therapeutics,” Stroka said. “But, first, we need to know what intracellular or extracellular cues cause AQP upregulation in the first place, and mechanistically how AQPs drive invasive cell migration. We are excited to explore these questions through this new NIGMS MIRA grant.”
This research is supported by the National Institutes of Health (NIH) National Institute of General Medical Sciences (NIGMS) Maximizing Investigators' Research Award (MIRA) (R35). Stroka’s previous work in this area was highlighted in Cell under the title “Water Permeation Drives Tumor Cell Migration in Confined Microenvironments.”
Published September 9, 2021