News Story
Creating the “Internet of Life”
University of Maryland researchers are investigating how to connect electronics with living biological systems. This idea may conjure visions from science fiction films, but an interdisciplinary team of researchers from the Robert E. Fischell Institute for Biomedical Devices and UMD’s Institute of Bioscience and Biotechnology Research (IBBR) have made very real strides in creating a functional medium of communication between these two different systems.
Recently published in Nature Communications, the researchers successfully connected E. coli bacterial cells to a machine. Using chemical reactions and genetic engineering to achieve this, the team’s paper demonstrates how electronic signals can control the biological processes of cells in real-time.
For several years, the labs of Fischell Institute Director and Fischell Department of Bioengineering (BIOE) professor William E. Bentley and IBBR Research Professor and Fischell Institute Fellow Gregory F. Payne have worked together to advance bioelectronic technology. They suggest that while our society has made transformative progress in some areas of bioelectronics—such as cardiovascular function, EKG, and defibrillators—there has been little advancement in using simple electronics to access molecular information—for example, measuring cholesterol. Only glucose is readily accessed, and the process to do so for individuals with diabetes can be complicated, time consuming, and very expensive. If we had simple electronic access to molecular information, the team argues, we would be in a completely different situation. "A long standing impediment to commercialized bioelectronics technology is the ability to successfully establish a seamless connection between biological systems and electronic devices," they say. "As with so many complex relationships, the core of the solution requires good communication—the successful exchange of information."
 "Biology and electronics are very different when it comes to how they communicate," explains Sally Wang (BIOE Ph.D. '23), a postdoctoral researcher in the Bentley Lab. Wang is co-lead author on this paper with Chen-Yu Chen (BIOE Ph.D. '23), a member of Bentley Lab, the Fischell Institute, and IBBR.
"Biology and electronics are very different when it comes to how they communicate," explains Sally Wang (BIOE Ph.D. '23), a postdoctoral researcher in the Bentley Lab. Wang is co-lead author on this paper with Chen-Yu Chen (BIOE Ph.D. '23), a member of Bentley Lab, the Fischell Institute, and IBBR.
"Electronics like your computers are designed so that electrons can pass through wires like a highway to spread information," says Wang. "Or if it's wireless—like our phones or Bluetooth—the information is sent out using electromagnetic waves. In biology, there aren’t free electrons moving through your body. So what do biological systems do to move those electrons? They transfer electrons using redox reactions."
Redox molecules can transport electrons from one place to another using reduction-oxidation (redox) reactions, chemical reactions that cause the gain and loss of electrons within cells.
Nearly six years ago, Bentley and Payne discovered these redox reactions to be a bridge in the communication gap between biological and electronic systems. Recognizing that redox molecules form the foundation for electron transfer in biology, Bentley and Payne have worked to engineer and manipulate biological redox networks for information transfer between the electronics of devices and the electronics of biology at multiple levels, including proteins, individual cells, and groups of cells. This multifaceted and interwoven connection between systems is what the team has coined the "Internet of Life."
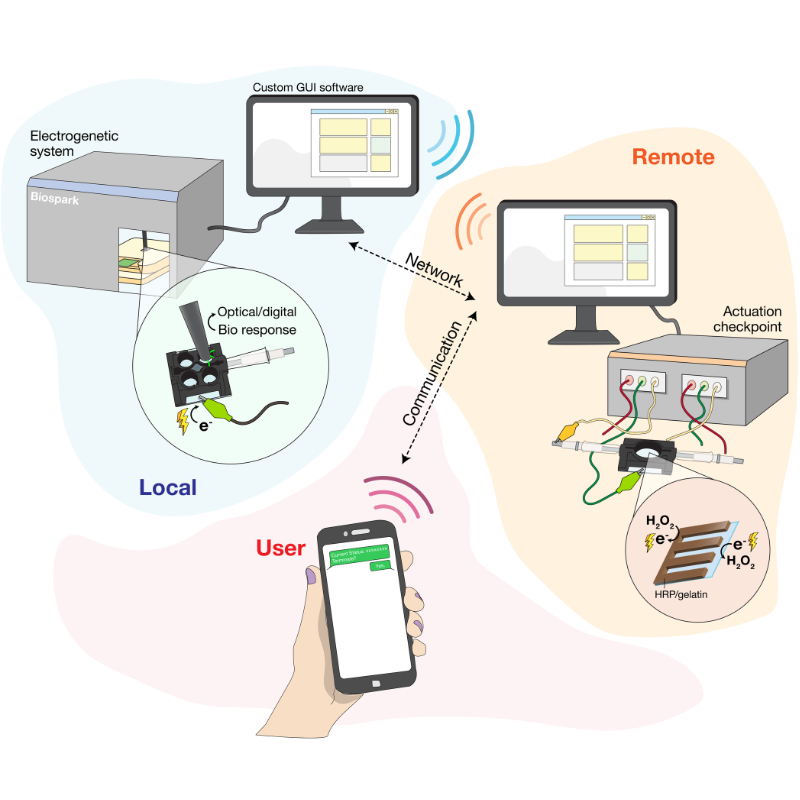 Building upon this research by Bentley and Payne, Wang and Chen have taken steps forward in establishing a functional communication bridge. In their paper, they demonstrate a closed-loop system where a cell’s biological activity can be monitored in real-time using electronic signals and, further, the cell’s genetic systems can be electronically controlled. The latter function is referred to as "electrogenetics," an approach that the UMD team introduced and that has since been adopted by several groups worldwide.
Building upon this research by Bentley and Payne, Wang and Chen have taken steps forward in establishing a functional communication bridge. In their paper, they demonstrate a closed-loop system where a cell’s biological activity can be monitored in real-time using electronic signals and, further, the cell’s genetic systems can be electronically controlled. The latter function is referred to as "electrogenetics," an approach that the UMD team introduced and that has since been adopted by several groups worldwide.
To establish the "Internet of Life," the team begins by strategically placing E. coli bacterial cells within a gel of polyethylene glycol, a coating that is also used to encapsulate drugs. Also touching the gel are specialized electrodes, which feed electricity to the gel to produce hydrogen peroxide. This peroxide becomes the "middleman of communication," Wang explains, helping to spur redox reactions and the transfer of electrons to the bacterial cells.
“Together, Sally and Chen-Yu's expertise allowed them to develop a transformative technology that would have not been possible without both their backgrounds, approaches, and work ethic. They did something that I had envisioned with great anticipation for quite some time. It took this magical melding of abilities and effort to make it all work.”
William E. Bentley, Fischell Institute Director
The bacterial cells used in this system have been engineered to accept electrons from the peroxide—which they otherwise would not have done—with the help of the cell’s own stress response. To engineer this behavior, the cells’ genes were altered with CRISPR technology, a revolutionary gene-editing and transcription control tool. The team endowed the E. coli cells' oxidative stress regulon with a CRISPR toolkit, enabling the bacteria to set off optical or electrochemical signals upon accepting the electrons from hydrogen peroxide. These output signals include green fluorescence from a green fluorescent protein that originated from jellyfish, and electrical currents, generated by redox-active antibiotics from Pseudomonas bacteria.
The fluorescence and electrochemical currents can be observed and recorded by BioSpark—the team’s newly created, cutting-edge bioelectronic device. BioSpark receives and digitizes these biological signals, and is able to give electronic commands back to the system, such as giving more stimulation to electrochemically make hydrogen peroxide so that the cells make more fluorescence and electrical currents.
This real-time computing allows for a feedback loop, in which current data are used to make on-the-spot decisions on controlling and maintaining the electrode-generated cell activity, establishing a closed "electro-bio-electro" communication loop. This novel system also has a cascaded control architecture, meaning multiple levels of control work together. One such level is that a human operator can supervise, approve actions, or even terminate the processes via text messaging—furthering the electronic means by which the cells are being controlled.
The team’s successful connection of bacterial cells to electronics, in addition to the demonstrated ability to electronically control cell behavior, has larger implications in addressing the grand challenges of today.
"What is transformative here is the activation using electronics. This opens doors for building completely new ways to connect information and data-rich technologies to biology," says Bentley. "There are myriad opportunities that could emerge from electrogenetics."
There are applications across healthcare, agriculture, environmental conservation, and beyond that now seem within the realm of possibility with continued research. A self-regulated device connected to the human body to read disease progression and administer drugs as needed. A “smart” farmland monitor that can telemetrically inform how to influence the microbiome in the field, how much pesticide and herbicide to use and when. Advanced sensing technology that can detect, analyze, and mitigate environmental pollutants.
For Wang, this success is only the first stop in her postdoctoral research. She is eager to replicate this success beyond prokaryotic cells—like the E. coli bacterial cells used in their paper—and successfully transfer this process to mammalian cells and eukaryotic systems like those in the human body. She also hopes to create a more intricate and sophisticated control system from BioSpark that will have more translatable applications in the medical and manufacturing fields.
Before they were lead authors, Wang and Chen came to UMD to pursue bioengineering graduate degrees from very different academic backgrounds. Wang had a B.S. in biochemistry, and Chen held a B.S. and MEng in chemical engineering. While Wang’s research focused on the biological aspects of molecular and cellular sciences, Chen had a more device-focused approach.
"Together, their expertise allowed them to develop a transformative technology that would have not been possible without both their backgrounds, approaches, and work ethic," says Bentley, who served as their faculty advisor during their graduate studies. "They did something that I had envisioned with great anticipation for quite some time. It took this magical melding of abilities and effort to make it all work."
Other authors of this study include Fischell Institute researchers John R. Rzasa, Chen-Yu Tsao, Eric VanArsdale, and Fauziah Rahma Zakaria, and IBBR members Jinyang Li and Eunkyoung Kim.
Published February 29, 2024









