News Story
Taking Aim at a Rare Lysosomal Storage Disease

BioE graduate student Janet Hsu and Biological Sciences Graduate Program (BISI) student Daniel Serrano, advised by Assistant Professor Silvia Muro (joint; Institute for Bioscience and Biotechnology Research) are the first authors of the paper, which was featured on the cover of the Journal of Controlled Release, one of the top publications focusing on drug and gene delivery, tissue engineering, and related diagnostics.
Fabry disease is a hereditary disorder that affects lysosomes, hollow, organ-like structures within cells that contain enzymes, specialized proteins that accelerate or enable chemical reactions. The lysosomes' job is to break down the lipids (fats), carbohydrates, and other proteins cells require for food, making them easier to digest. Lysosomal storage disorders are those in which a genetic defect results in a shortage or absence of a particular enzyme the lysosomes need in order to function. As unprocessed material accumulates in the cells, they swell and rupture.
In the case of Fabry disease, the body fails to produce enough α-galactosidase (α-Gal), an enzyme that breaks down certain sugar residues attached to fat molecules. Fabry disease damages the cardiovascular and renal systems, leading to kidney and liver failure, heart disease, skin and vision problems, pain, and premature death.
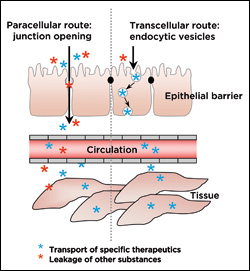
Currently, enzyme replacement therapy (ERT) is used to treat patients with the condition. Large doses of medication are required because only a small percentage of the α-Gal administered is successfully delivered to the lysosomes of the cells. Hsu, Serrano and their colleagues devised a new, more effective kind of ERT that uses a targeted delivery system to bring α-Gal to the affected cells and make it easier for the cells to ingest it.
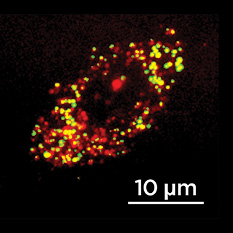
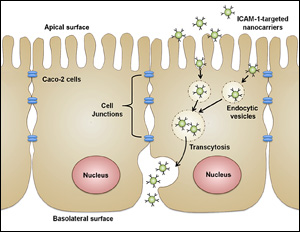
Hsu attached α-Gal to a nanocarrier coated with anti-ICAM, a molecule designed to fit perfectly, like a neighboring puzzle piece, into a protein called intercellular adhesion molecule 1 (ICAM-1), which is produced by the endothelial cells that line the interior surfaces of blood vessels throughout the body. As the nanocarriers move through the cardiovascular system, the anti-ICAM and ICAM-1 molecules lock together, pulling the α-Gal to the cells and depositing the enzyme into their lysomes so normal cellular function can resume. Hsu found that the use of the anti-ICAM-coated nanocarriers improved amount of α-Gal successfully delivered to and taken up (ingested) by endothelial cells, rendering them capable of breaking down the aberrant lysosomal storage. The results suggest that the targeting system could improve the efficacy of ERT treatment and reduce overall costs. Moving forward, Hsu and her colleagues will work to ensure the nanocarriers load with a consistent amount of α-Gal so treatments can be standardized and properly measured.
The Journal of Controlled Release cover story essay on the article by editor-in-chief Kinam Park (Purdue University) highlights the fact that, while drug delivery systems are most often developed to carry small and poorly soluble drugs, the Muro Group's is an example in which they have been designed to carry a relatively large and hydrophilic drug, a therapeutic enzyme, representing a relatively unexplored strategy. Park also emphasizes the relevance of the multiple parameters measured in the work, and describes them as an example to follow in studies dealing with targeted drug delivery systems.
This work has been funded by the Maryland Department of Business and Economic Development's Nanobiotechnology Program, the Minta Martin Foundation, the American Heart Association, and the National Institutes of Health.
For More Information:
Visit Professor Muro's homepage »
Janet Hsu, Daniel Serrano, Tridib Bhowmick, Kishan Kumard, Yang Shene, Yuan Chia Kuoa, Carmen Garnachoc, and Silvia Muro. "Enhanced endothelial delivery and biochemical effects of α-galactosidase by ICAM-1-targeted nanocarriers for Fabry disease." Journal of Controlled Release 149 (2011) 323–331. Abstract »
Kinam Park. "Enhanced delivery to endothelial lysosomes by ICAM-1-targeted nanocarriers." Journal of Controlled Release 149 (2011) 207–208. Cover story essay. PDF »
Published April 19, 2011