News Story
Advisory Board Member Pinchuk's Co. Develops Device to Treat Eye Disorders
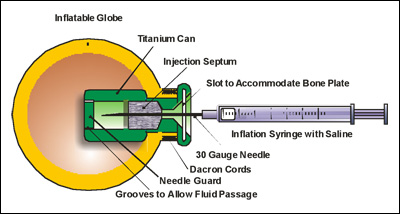
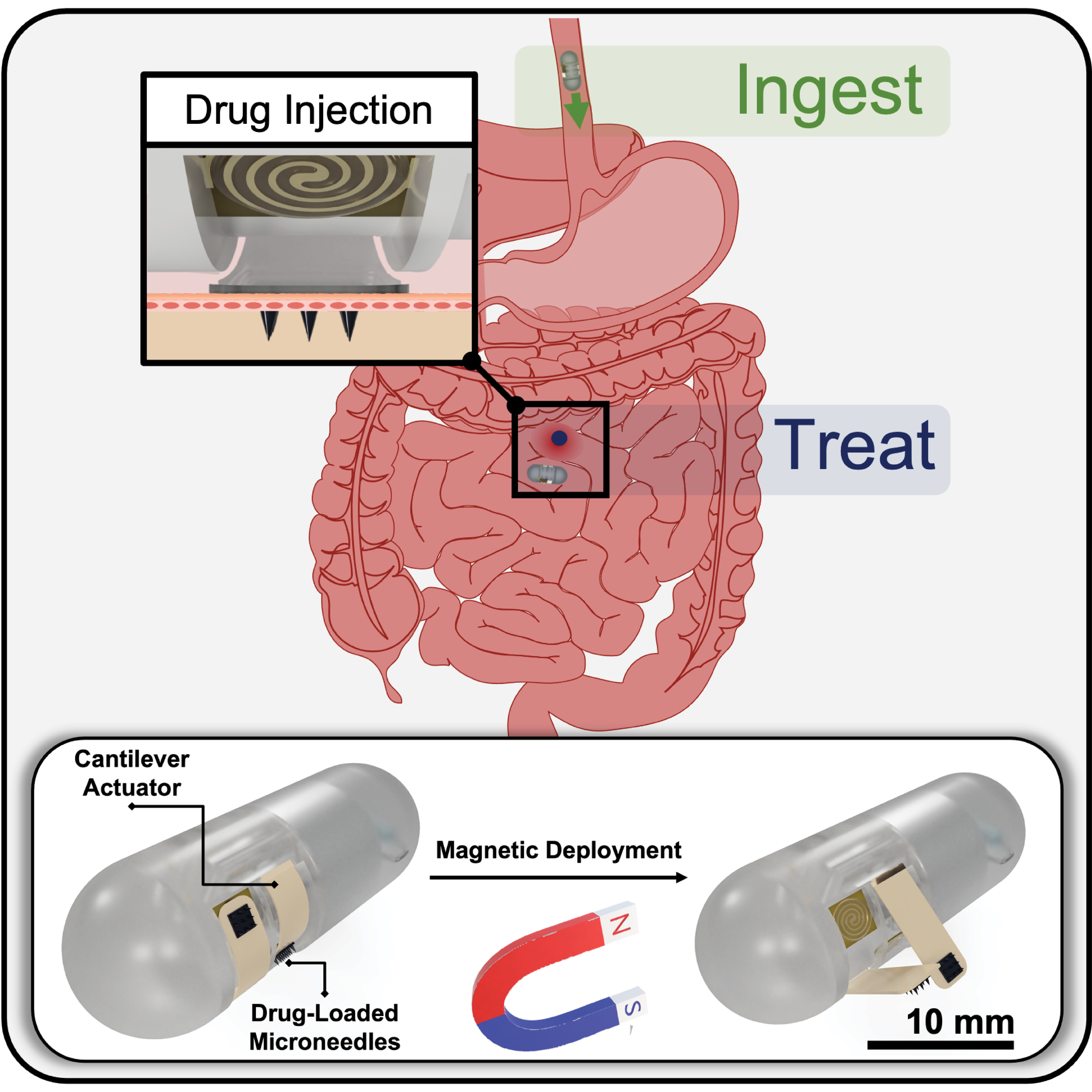
Diagram of the Orbital Tissue Expander (OTE), which shows how injected fluid gradually expands an implant to assist in the normal development of the skull as a child grows.
Thirteen years ago, when doctors told Diann O'Riordan of Claremore, Ok., that her three-week old son was diagnosed with microphthalmia, she had no idea what to do.
Like most parents of children born with the disorder, O’Riordan knew nothing about the rare birth defect that causes blindness in infants. "I was in total shock and disbelief," O'Riordan writes on a Web site that she later developed for parents in her situation. "How? Why? What did I do wrong?"
Almost thirteen years later, Jacob O'Riordan is a regular kid. He watches the Disney channel and surfs the Internet—with an artificial eye.
Anophthalmia and microphthalmia (A/M) are most often congenital disorders that lead infants to be born either completely without an eye (anophthalmia) or with an abnormally small eye (microphthalmia). Both disorders progress to blindness and, if left untreated, to facial deformities. The disorders can affect one or both eyes.
According to the International Children's Anophthalmia & Microphthalmia Network (ican), which provides information and support to families like the O’Riordans, "A/M is rare, but the exact incidence is unknown." A study in England showed the incidence of A/M was "1.0 per 10,000 births," the organization's Web site said.
Though the cause of A/M in infants is unknown, ican's Web site lists "inherited genetic mutations, sporadic genetic mutations, chromosome abnormalities and prenatal environmental insult" as potential risk factors.
While there is no cure or reversal for A/M, treatment is necessary. With no eye to uphold the structure of the facial bones, the skull may begin to shift during the child’s growth. To prevent and correct these deformities of the skull, doctors implant a device in place of the eye as soon as possible.
According to ican, the traditional device, called a conformer, is a "plastic ball-like device…that can be placed inside the [eye socket] to help support the growth of the eye socket and the bones in the face." Conformers are specially molded to fit the patient's eye, ensuring that as the infant grows, his face will develop normally.
While conformers are successful in the prevention of facial deformities, they are no longer the most practical method, said Dr. David Tse, an orbital surgeon in Miami. Because the child grows quickly in his early stages of life, conformers must constantly be replaced to keep up with this growth.
Tse said that a major disadvantage of conformers is the recurring trauma and pain caused in patients due to the multiple surgeries. Also, if one conformer is made too large or too small for the eye socket, other deformities may occur, defeating the purpose of the device. In O'Riordan’s case, her son received six different conformers by the time he was seven months old.
Other major concerns for many families are the aesthetics of the conformers.
"The only real problem that I had was with the public," O'Riordan wrote. "When Jake started wearing his conformers, we just got stares. No one would talk to him or play with him."
To solve these problems presented by the conformers, Tse worked for three years with a team of engineers to design and develop an improved device. In December 2007, Tse performed the first successful implantation of the new device, the Orbital Tissue Expander (OTE).
Instead of a hard, acrylic shell, the OTE is made of a softer, non-biodegradable material. Unlike the conformer, the OTE is not removed. It is instead injected with fluid and inflated as the infant grows. The OTE enables doctors to control inflation and deflation, avoiding the dangerous, painful and expensive risks of the device not fitting properly.
"The device stimulates bone growth in the face by applying constant pressure," said Marcia A. Orozco, senior engineer at Innovia LLC, the firm that manufactures the OTE. "As the patient grows, the OTE is periodically inflated," said Orozco. "This stimulates bone growth around it."
Tse and Orozco agree that the OTE has been very successful in patients with A/M. In spite of having such a small market, the OTE has been used throughout the United States and in the Middle East, where, according to Tse, A/M is more prevalent.
"We are just getting started and the market is small," said Anne Bohsack, Vice President of Sales and Operations at FCI Ophthalmics, Inc., which distributes the OTE at $2,500 each. "We have sold 12 to 14 units thus far, but hope to grow that number substantially going forward."
According to an article by Wendy W. Lee, M.D., M.S. on OphthalmologyWeb.com, the OTE is the best option for children who are dependent on a growing eye for socket development.
"Use of this implant avoids the necessity for repeat trips back to the operating room," wrote Lee. "This is a fantastic implant that can be used in anophthalmic children."
Article by and courtesy of Kerri Pinchuk.
For More Information:
Published July 22, 2010